Background: Exagamglogene autotemcel (exa-cel) is a non-viral cell therapy designed to reactivate fetal hemoglobin via ex vivo CRISPR-Cas9 gene-editing of autologous CD34+ hematopoietic stem and progenitor cells (HSPCs) at the erythroid-specific enhancer region of the BCL11A gene in patients (pts) with severe sickle cell disease (SCD). We report that in a pre-specified interim analysis, the pivotal CLIMB SCD-121 trial of exa-cel met primary and key secondary endpoints.
Methods: CLIMB SCD-121 is an ongoing, 24-mo, phase 3 trial of exa-cel in pts age 12-35y with SCD and a history of ≥2 VOCs/y in 2y prior to screening. Primary efficacy endpoint is proportion of pts free of severe VOCs for ≥12 consecutive months (mos) (VF12); key secondary efficacy endpoints are proportion of pts free from inpatient hospitalization for severe VOCs for ≥12 consecutive mos (HF12) and proportion of pts free from severe VOCs for ≥9 consecutive mos (VF9). Evaluable pts for VF12 and HF12 had ≥16 mos follow-up after exa-cel infusion; pts evaluable for VF9 had ≥12 mos follow-up after infusion. Evaluation of primary and key secondary endpoints began 60 days after last RBC transfusion for post-transplant support or SCD management. Pts completing trial enrolled in long-term follow-up Study 131. Mean (SD) are shown except where noted.
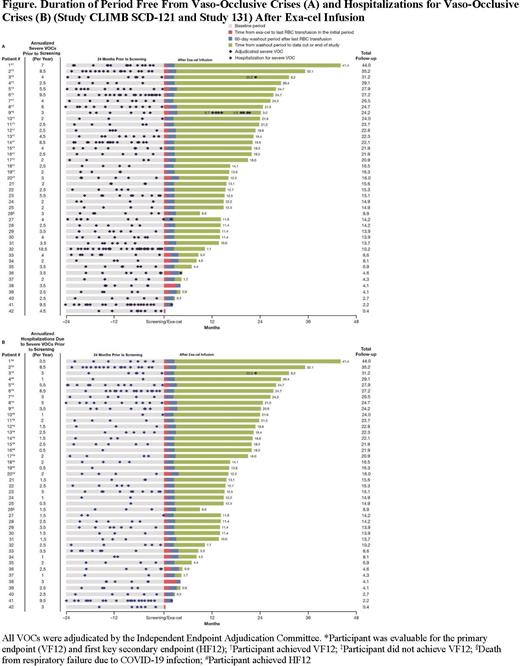
Results: As of 10 Feb 2023, 42 pts with SCD (age 21.2[range 12-34]y; 12[28.6%] age ≥12 to <18y; 4.2 VOCs/y at baseline) received exa-cel. Following infusion, all pts engrafted neutrophils and platelets (median 27 and 34.5 days, respectively). 19/20 (95.0%) pts evaluable for primary endpoint were free of VOCs for ≥12 consecutive mos (VF12; 95% CI, 75.1% to 99.9%; P<0.0001), 20/20 (100%) were free from hospitalizations for VOCs for ≥12 consecutive mos (HF12; 95% CI, 83.2 to 100.0; P<0.0001), and 29/30 (96.7%) were free of VOCs for ≥9 consecutive mos (VF9; 95% CI, 82.8 to 99.9; P<0.0001). In pts achieving VF12, VOC free duration was 21.8 (range 12.3-41.4) mos; 18 pts remained VOC free through follow-up and 1 pt had an adjudicated VOC in the setting of parvovirus infection ~22.8 mos after exa-cel; pt recovered fully and has since been VOC free (Fig). For all pts, total Hb was 12.1 g/dL at Month 3 and was maintained at ≥11.0 g/dL from Month 6 onward; HbF was 36.0% at Month 3 and was generally maintained at ≥ 40.0% from Month 6 onward with pancellular distribution (≥95% RBCs expressing HbF). Proportion of edited BCL11A alleles was stable over time in bone marrow CD34 + and peripheral blood nucleated cells. 36/39 pts with ≥60 days follow-up after last RBC transfusion (including those not yet evaluable) remained VOC free (up to 41.4 mos; Fig). Quality-of-life (QOL) measures showed clinically significant improvements from baseline.
All pts had ≥1 adverse event (AE), most were Grade 1 or 2; 40 (95.2%) pts had AEs of Grade 3 or 4 severity. Most common AEs were nausea (66.7%), stomatitis (61.9%), febrile neutropenia (52.4%), headache (52.4%), and vomiting (52.4%). Most AEs and serious AEs (SAEs) occurred within first 6 mos after infusion. No pts had SAEs considered related to exa-cel. As previously reported, 1 pt died from respiratory failure due to COVID-19 unrelated to exa-cel. There were no study discontinuations or malignancies.
Conclusions: The CLIMB SCD-121 trial met primary and key secondary endpoints, with exa-cel treatment resulting in early and sustained increases in Hb and HbF leading to elimination of VOCs in 95% of pts, elimination of inpatient hospitalization for VOCs in 100% of pts and improved QOL. Safety profile of exa-cel was generally consistent with myeloablative busulfan conditioning and autologous transplantation. These results show exa-cel has the potential to deliver a one-time functional cure to pts with severe SCD.
Disclosures
Frangoul:Editas Medicine: Consultancy; Rocket Pharmaceuticals: Consultancy, Other: Member of DSMB for a study; Jazz Pharmaceuticals: Speakers Bureau; Vertex Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sharma:Editas Medicine: Consultancy; RCI BMT/NMDP: Honoraria, Other: Clinical Trial Medical Monitor; Medexus Inc: Consultancy; Vertex Pharmaceuticals: Consultancy, Other: Clinical Trial Site PI; Sangamo Therapeutics: Consultancy; CRISPR Therapeutics: Other: Clinical Trial Site PI, Research Funding. Mapara:Crispr/vertex: Consultancy; Incyte: Consultancy; Bluebird bio: Consultancy. Liem:Bluebird Bio: Research Funding; NIH/NHLBI: Research Funding; NIH/NCATS: Research Funding; Vertex: Research Funding; Editas: Research Funding; Global Blood Therapeutics: Research Funding. Telfer:Apopharma: Other: Clinical trial activity, Speakers Bureau; Terumo: Speakers Bureau; Pfizer: Other: Advisory board; Clinical trial activity; Data monitoring committee; ; Napp Pharmaceuticals: Other: Clinical trial activity; Celgene: Other: Clinical trial activity; Kyowa Kirin Limited: Other: Investigator-led funding; Novartis: Other: Advisory board; Clinical trial activity; ; bluebird bio: Other: Advisory board; Investigator-led funding;. Shah:Vertex: Membership on an entity's Board of Directors or advisory committees. Rondelli:Vertex: Other: Steering Committee. Meisel:Miltenyi Biotech: Research Funding; medac: Consultancy, Research Funding, Speakers Bureau; Gilead/KITE: Research Funding; Novartis: Consultancy, Research Funding, Speakers Bureau; CELGENE BMS: Consultancy, Research Funding, Speakers Bureau; Bluebird Bio: Consultancy, Speakers Bureau; CRISPR Therapeutics: Consultancy, Research Funding, Speakers Bureau; Vertex: Consultancy, Research Funding, Speakers Bureau. Lobitz:AddMedica: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Patient Booklet and educational papers; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; BlueBird Bio: Membership on an entity's Board of Directors or advisory committees, Other: Lecture; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee; German National Disease Management Program: Other: Spokesperson for children and adolescents with sickle cell disease; German National Treatment Guideline: Other: Corresponding author of the treatment guideline for children and adolescents with sickle cell disease. de Montalembert:Addmedica: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: ASH meeting 2022 support; Novartis: Membership on an entity's Board of Directors or advisory committees. Steinberg:NIH: Research Funding; Fulcrum Therapeutics: Consultancy; ASH: Other: Lecture at HEM ASH Whiteboard Symposium, Speakers Bureau; Imara Therapeutics: Membership on an entity's Board of Directors or advisory committees. Walters:Ensoma, Inc: Consultancy; Vertex Pharmaceuticals: Consultancy; BioChip Labs: Consultancy, Other: Medical Director; AllCells, Inc: Consultancy, Other: Medical Director. Bower:Vertex Pharmaceuticals: Current Employment. Imren:Vertex Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Simard:Vertex Pharmaceuticals: Current Employment. Xuan:Vertex Pharmaceuticals: Current Employment. Zhou:Vertex Pharmaceuticals: Current Employment. Morrow:Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Current Employment, Membership on an entity's Board of Directors or advisory committees. Hobbs:Vertex Pharmaceuticals: Current Employment. Grupp:Cabaletta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellectis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; CBMG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Kite: Research Funding; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Vertex: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.